Last Updated on April 20, 2020
 If you ever worked for a big company, then maybe you know those “team building” sessions which consisted mainly of playing all kind of games, more or less stupid, followed by wise debriefings, meant to consolidate the team and to make its members to better know each other. Yet, there was a series of games that I found interesting: the ones in which you are given several items and you have to complete a task using only those items.
If you ever worked for a big company, then maybe you know those “team building” sessions which consisted mainly of playing all kind of games, more or less stupid, followed by wise debriefings, meant to consolidate the team and to make its members to better know each other. Yet, there was a series of games that I found interesting: the ones in which you are given several items and you have to complete a task using only those items.
This is one example of smart game (the best part comes at the end):
Get that coin out of the water without getting yourself wet and without moving the plate
Items given:
– one plate containing a small quantity of water
– one coin, on the bottom of the plate, covered by water
– one candle (this can come from your own candle making production, if you have this hobby)
– one glass
– one lighter
Briefing: you are not allowed to move the plate.
If you want to try it for yourself, stop reading. If you just want to see how it’s done, then here you are:
Step 1: put the candle in the water, not very close to the coin, and light it.

Step 2: Put the glass upside down over the lit candle.

Step 3: Wait. The candle extinguishes after the oxygen in the glass is burnt, then you will see the water level slowly raising inside the glass. Soon all the water in the plate gets sucked inside the glass, so you can safely take the coin.
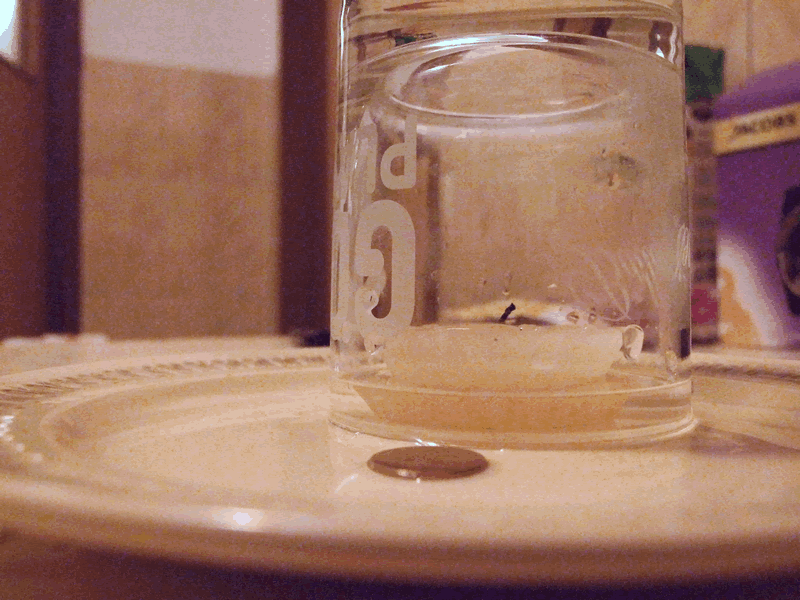
For making sure you don’t get in touch with water, you can wait a few minutes until the plate and the coin get dry.
If you want to amaze your friends with this trick, try it before, in order to adjust the amount of water you need to use.
The best part comes now: you are challenged!
Contest:
Three of the readers who guess what is the phenomenon that makes this happen and blog about it linking to this post with the anchor phrase cool tips and tricks win one year free hosting with Dreamhost for a domain, with MYSQL database included. I reserve myself the right to choose the winning answers. The other participants who answer correctly will get a free link from this site (PR5).
The contest starts now and it ends on April 30th at midnight (your timezone).
Good luck everybody, and remember the rules:
1. know the answer
2. blog about it
3. link to this post with the anchor text “cool tips and tricks”
4. leave a comment on this page, so I know that you are in




This is a cool trick! I have no idea about what kind of magic lies behind, but I’ll show it to my friends at the next party 🙂
Maybe the flame consumed the oxygen, forcing the water to fill the gap?
I surely could use one year of hosting 😉
I’m sorry, Sunny, you were close, but this is not the answer. You can try again, if you think of something else.
the air is hot around the flame, when puting the glass, a lot of hot air is captured
a time after the candle extinguishes, the air get cold down, as the volume and mass remain the same, the pressure inside goes down too
the pressure inside will be less than the pressure outside, and the water is pushed inside by the outside pressure
Thank you, Mihai, this could be a possible explanation. Don’t forget to link to this post from your blog before April 30th.
Anyone else has a better one?
The hot air inside the glass is less dense (and thus at a lower pressure) than the cooler outside air. The difference creates suction, which pulls the water inside the glass.
Thank you, Julie. You are in. Don’t forget to put the link on your blog :).
Ok here is my answer hopefully it qualifies.
The basic premise is the change in air pressure. When the open end of the cup is submerged in the water it creates a type of water seal preventing additional air from entering the space. When the candle is lit it begins to heat up the air around it, burning the oxygen up and according to Boyle’s law, when a gas (air) is heated its density is reduced. (This is the motive “force” behind hot air balloons by the way). The reduced density of the hot air in a closed container creates a vacuum.
The air pressure being exerted on the water outside of the jar is greater than that being exerted inside the jar so the water moves towards the path of less resistance and pushes itself inside. Technically if there were enough water and the candle was allowed to burn itself out, the water would fill approximate 1/5th of the volume available inside the jar since air is almost 20% oxygen.
Hope that works for you. Thanks for the challenge!
Hey if I do win, I don’t really need the Dreamhost hosting but I would love to use it as a joint prize for a challenge on my blog if you agree. By joint I mean “Prize supplied by AllTipsAndTricks.Com” or something like that.
Can’t wait to see if I won! 🙂
Thank you Susan, you are in. There are only a few more days to go, then I’ll announce the winners. Your idea to use the prize for one of your contests is great, I fully agree with that.
I posted my answer at http://www.savvyaffiliate.com/Blog/uncategorized/cool-tips-and-tricks/
Synopsis (I’d copy and paste but don’t want the duplicate content penalty )
1) Jar holds a finite amount of air
2) Candle heats up air
3) PV=nRT dictates that under finite volume hotter air increases pressure
4) High pressure air bubbles out of the jar
5) Candle burns out => air cools down
6) Air pressure in jar decreases
7) Water in jar rises until the pressure difference between the jar and the atmosphere equals the height of the column of water * density of water * gravity
Thank you, Scott, you’re in! A few more days to go until the contest ends.
Susan.. when a gas is heated it expands this would cause it to bloe air out of the cup its nothing to do with that.. its simply the oxygen being burned up leaving empty space (apart from the other gases in the air) this empty space causes a vacum and a vacum doesnt like being a vacuum so it sucks stuff in.. a man made black hole >.
Ha, ha! nice observation, Anonymous! However, the oxygen percentage in the air is so low, that even if it burns, that’s not enough to make the water climb in the glass. When the air is heated, there is less air in the glass, correct? Then, after the candle is extinguished, the air in the glass gets colder, thus decreasing its volume. As you just said, the vacuum it causes does not like to be a vacuum, so it sucks stuff in. 🙂